New, promising flax varieties for Manitobans
Manitoba flax farmers (and upcoming flax farmers) will soon have full access to a new flax variety, CDC Esme. As of spring 2025, CDC Esme is available to seed producers across the province, as long as they have been able to access it from seed dealers.
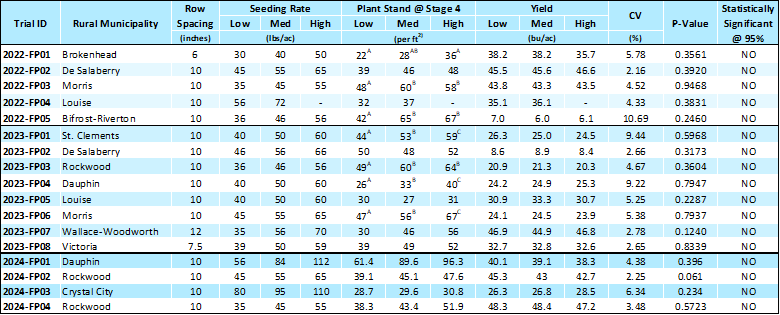
As stated in the Secan Technical Bulletin for the variety, CDC Esme is a large seeded, high yielding brown seeded flax variety with good lodging resistance and late maturity. Esme proves to be a strong yielder in MCVET trials across several site years, with oil content similar to check varieties. Statistics show an average of 2 cm shorter than CDC Glas, which is a beneficial trend in Manitoba, where farmers are trying to reduce flax straw and residue.
Dr. Bunyamin Tar’an and his team at the Crop Development Centre in Saskatoon developed CDC Esme and have several more flax lines that have strong potential for registration on the Canadian Prairies. Most recently, Tar’an had an experimental flax line that has been recommended for registration via Canadian Food Inspection Agency. This line, currently named FP 2608, will undergo a registration process and could likely be available for seed production in the next two years.
FP 2608 has been a promising yielder in the Flax Co-op trials performed in Manitoba and is a consistently strong yielder. It appears to be quite tolerant of pasmo infections in past reports, and oil content and yield has been ranked very high amongst other varieties and lines. Tar’an has noted that FP 2608 had considerably strong performance in the more northern Co-op trials in Manitoba, which perhaps displays its ability to thrive in less diverse rotations.
MCA is very proud of our relationship with Dr. Tar’an and are very excited about his future work in flax breeding. Some new work that he is working on includes “Accelerated Breeding Strategy for Flax Improvement” which looks at developing inbred populations in a shorter term than is current reality. This would mean that future varieties could potentially be brought to the market more quickly and readily than they have in the past. Another highlight of Dr. Tar’an’s work is that he is committed to reducing straw content while increasing seed production in future varieties. He has had to delve back in time and use flax’s wild ancestor, Linum bienne. By using historial genetics, Tar’an can benefit by breeding their old, shorter varieties with new modern varieties that have bushier plant structure, therefore, higher yielding qualities and improved harvestability.
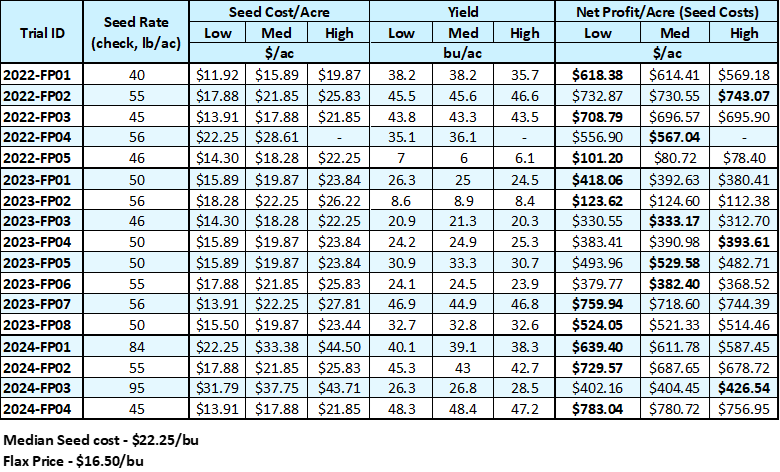
Flax can have a strong future on the Prairies and Manitoba Crop Alliance is supporting that possibility for our farmers. It is a crop that requires patience and consideration, which we believe Manitoba farmers are great at and can help us solidify the flax industry on the Prairies. Discussions with farmers that have been growing flax successfully for several years tell us that it is one of the top most profitable crops on their farms because they are willing to put an extra effort in to the crop. In this case, it doesn’t mean inputs, directly. These farmers are treating flax like the special crop that it is; slowing down and using precision and sustainable practices to support the germination of a tiny seed into a beautiful and profitable crop.
Please contact the MCA’s Agronomy Extension Specialist for Special Crops if you are considering growing flax or currently work with flax and are interested in more agronomic information.